Long QT syndrome occurs due to abnormalities in cardiac sodium and potassium ion channels that result in a propensity to fatal ventricular arrhythmias. Ventricular arrhythmias are precipitated by stimuli that involve the production of adrenalin, such as physical exercise (long QT type 1); fear or sudden or loud auditory noises – as in Kasia Ber’s case where the stimulus to the fatal ventricular rhythm disturbance may have been the telephone alarm ring – and intense emotion (long QT type 2), resulting in dizziness, syncope and transient seizures due to cerebral hypoperfusion – the arrhythmia means that heart does not pump enough blood to the brain.
Many cases of long QT syndrome are familial, being inherited as an autosomal dominant or excessive trait. Treatment of congenital long QT syndrome consists of high-dose beta-blockers.
In order to understand why sudden death can happen, it helps to understand how the heart works. The heart is a specialised muscle that contracts regularly and continuously, pumping blood to the body and the lungs. The pumping action is caused by a flow of electricity through the heart that repeats itself in a cycle. If this electrical activity is disrupted – for example by a disturbance in the heart’s rhythm, known as an ‘arrhythmia’ – it can affect the heart’s ability to pump properly.
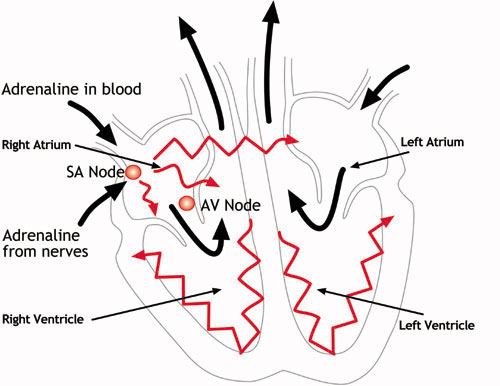
The heart has four chambers – two at the top (the atria) and two at the bottom (the ventricles). The normal trigger for the heart to contract arises from the heart’s natural pacemaker, the SA node, which is in the top chamber – see the diagram below. The SA node sends out regular electrical impulses causing the atrium to contract and to pump blood into the bottom chamber (the ventricle).
The electrical impulse then passes to the ventricles through a form of ‘junction box’ called the AV node (atrio-ventricular node). This electrical impulse spreads into the ventricles, causing the muscle to contract and to pump blood to the lungs and the body. Chemicals which circulate in the blood, and which are released by the nerves that regulate the heart, alter the speed of the pacemaker and the force of the pumping action of the ventricles. For example, adrenaline increases the heart rate and the volume of blood pumped by the heart.
How the heart functions electrically
The heart’s natural pacemaker – the SA node – sends out regular electrical impulses from the top chamber (the atrium) causing it to contract and pump blood into the bottom chamber (the ventricle). The electrical impulse is then conducted to the ventricles through a form of ‘junction box’ called the AV node. The impulse spreads into the ventricles, causing the muscle to contract and to pump out the blood. The blood from the right ventricle goes to the lungs, and the blood from the left ventricle goes to the body.
When an ECG reading is taken, the first short upward notch of the ECG trace is the “P wave” that indicates that the atria (the two upper chambers of the heart) are contracting to pump out blood. The next part of the tracing is a short downward section connected to a tall upward section. This is the “QRS complex” that indicates that the ventricles (the two lower chambers of the heart) are contracting to pump out blood. The next short upward segment is the “ST segment” that indicates the amount of time from the end of the contraction of the ventricles to the beginning of the rest period before the ventricles begin to contract for the next beat. The next upward curve is called the “T wave” which indicates the resting period of the ventricles.
The ion channelopathies are rare disorders of the DNA code (“mutations”) in specific genes that can cause sudden death due to heart rhythm disturbances. The genes in question produce proteins found mainly on the outside of cells that regulate electrical activity. Not detectable at post mortem, they can be detected by lengthy tests on heart tissue. The proteins involved in long QT syndrome are 2 of the potassium “channels” that regulate the behaviour of potassium ions moving from inside to outside the cell. A sodium “channel” that regulates the behaviour of sodium ions moving outside to inside the cell is also involved.
In long QT syndrome the potassium channels do not behave as efficiently as normal or the sodium channel over-activates resulting in electrical disturbance in the cell called prolonged repolarisation – i.e. recharging of the electrical system after each heart beat. The duration of the QT interval is a measure of the time required for depolarisation and repolarisation to occur. In long QT syndrome, the duration of repolarisation after each heartbeat is longer than normal, leaving the person vulnerable to a very fast, abnormal heart rhythm (an ‘arrhythmia’) known as ‘torsade de pointes’. When this rhythm occurs, no blood is pumped out from the heart, and the brain quickly becomes deprived of blood, causing the usual symptoms of sudden loss of consciousness (syncope) and, in some cases, sudden death.
Kasia’s Case
In the present case, Kasia Ber’s abnormal heart rhythm was stimulated by sudden noise. When this rhythm occurs, no blood is pumped out from the heart, and the brain quickly becomes deprived of blood, causing the usual symptoms of sudden loss of consciousness (syncope) and, as in this case, sudden death. As the condition is genetically mediated, it can be inherited.
Kasia Ber’s maternal grandfather died of arrhythmia; her mother was diagnosed with long QT syndrome but the diagnosis was not followed up within her family; Kasia’s maternal aunt died of arrhythmia; and, following Kasia’s death, that aunt’s two children were diagnosed with long QT syndrome. Those small points do not reflect cutting-edge medicine but the simple exercise of common sense. The condition can be treated by the use of relatively inexpensive drugs.
Not knowing anything about her heart condition, Kasia had consulted her general practitioner because she was having ‘funny turns’. The ECG that he carried out 11 days before she died showed that she had that hereditary heart condition but the diagnosis was not made, and she was not treated. Since her death, the condition has been found in 2 of her cousins. As a result they can be afforded treatment with inexpensive beta-blocker drugs.